Abstract
Background: We previously reported (Locatelli et al., Blood 2017) that TBdepl-haploHSCT is a suitable and effective option for children with AL lacking an HLA-compatible donor and/or needing an urgent allograft. This type of transplant is characterized by a low incidence of both acute and chronic graft-versus-host disease (GvHD) and by low non-relapse mortality (NRM), translating into survival outcomes comparable to those of patients transplanted from an HLA-compatible donor. Here we report an update on a cohort of 213 children with AL of our study, with a minimum follow-up of 180 days after the allograft.
Patients and methods: Between October 2010 and January 2022, 213 children with AL received TBdepl-haploHSCT from an HLA-partially matched relative at Ospedale Pediatrico Bambino Gesù in Rome, Italy. All patients were in morphological complete remission (CR) and were prepared to TBdepl-haploHSCT using a fully-myeloablative conditioning regimen including a combination of total body irradiation (TBI) and/or cytotoxic drugs. All patients received also anti-T-lymphocyte globulin (ATLG) before transplantation (12 mg/kg total dose, from days -5 to day -3) to modulate bi-directional donor/recipient alloreactivity and rituximab (200 mg/sqm) on day -1 to prevent post-transplantation EBV-induced lymphoproliferative disorders (PTLD). No patient received any post-transplant pharmacological GvHD prophylaxis. Data were analyzed as of July 15, 2022.
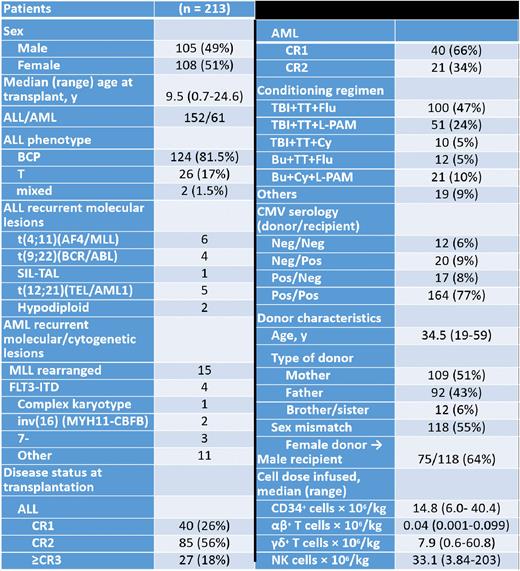
Results: Table 1 reports details on characteristics of patients included in the analysis. The median follow-up of surviving patients is 47.6 months (range: 6 months - 11.7 years). Two hundred and eight patients (98% of the total number) engrafted with a median time to neutrophil and platelet recovery of 13 (range 9-24) and 11 (range 7-23) days, respectively. Only 5 patients experienced graft failure; all were rescued with a second allograft. Eleven out of the 213 patients (5%) died of transplant-related complications (4 because of idiopathic pneumonia, 2 each because of disseminated adenovirus infection and thrombotic microangiopathy and 1 each of cardiac insufficiency, combined CMV/rhinovirus pneumonia and sepsis from Pseudomonas aeruginosa), the 5-year cumulative incidence of NRM being 5.2% (95% CI, 2.8-8.8). Forty-four patients relapsed at a median time of 192 days (range 56-1711) after HSCT, the 5-year cumulative incidence of relapse being 22.7% (95% CI, 16.9-29.2). Cumulative incidence of grade II-III acute GvHD was 14.7% (95% CI 10.6-20.3). Three patients and 1 developed gut and liver GvHD, respectively, while for all other patients skin was the sole organ involved. Fourteen out of the 193 patients at risk developed chronic GvHD, in all cases of limited severity, the cumulative incidence of this complication being 8.1% (95% CI 4.8-13.3). The 10-year probability of overall and leukemia-free survival (LFS) were 75.4 (95% CI 68.6-80.9) and 71.6% (95% CI 64.4-77.6), respectively. In univariate analysis, disease status at transplantation (CR1 vs CR2 vs CR>3), use of TBI during the preparative regimen and age at transplant above the median value (9.5 years) were associated with better patient's outcome, mainly because of a reduced incidence of relapse. Notably, among TBI-based regimens, the use of thiotepa and fludarabine was associated with an improved DFS as compared to other combinations (82.4% vs 63.9%, p=0.01). In a multivariable model for LFS including also gender and type of disease, only TBI (hazard ratio (HR) 0.38 (95% CI, 0.17-0.85, p=0.01) and disease status at HSCT (HR 1.72 (95% CI, 1.05-2.79, p=0.02) remained statistically significant. The 10-year GvHD/relapse-free survival was 65.1% (95% CI 57.7-71.4). The median CD3+ cell count on day +30, +90, +180 and +360 were 140, 185, 533 and 1058 /mcl, respectively.
Conclusions: TBdepl-haploHSCT is becoming a widespread haplo platform for pediatric patients affected by both malignant and non-malignant disorders. This cohort of homogeneously treated patients, the largest reported so far with a median follow-up of almost 4 years, confirm once more the effectiveness and safety of this approach. Furthermore, this analysis identified subgroups of patients (e.g., those in CR>3) for whom post-transplant strategies aimed at reducing the risk of relapse (the main cause of treatment failure) are desirable.
Disclosures
Merli:SOBI: Honoraria, Membership on an entity's Board of Directors or advisory committees; JAZZ Pharmaceuticals: Honoraria. Locatelli:JAZZ PHARMACEUTICALS: Speakers Bureau; SOBI: Speakers Bureau; MILTENYI: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MEDAC: Speakers Bureau; SANOFI: Membership on an entity's Board of Directors or advisory committees; BLUEBIRD BIO: Speakers Bureau; TAKEDA: Speakers Bureau; GILEAD: Speakers Bureau; AMGEN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NEOVII: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal